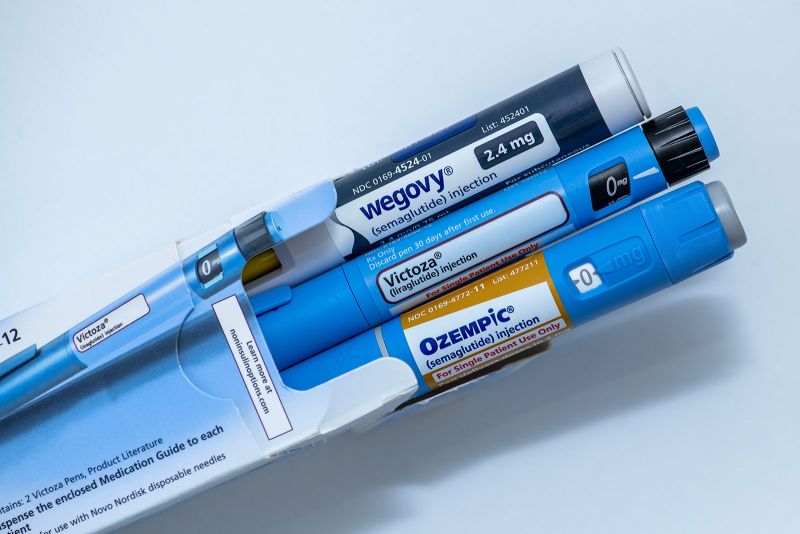
The US Food and Drug Administration on Monday approved a generic version of the daily injectable GLP-1 medicine liraglutide for people with type 2 diabetes, opening the door for lower-priced options to reach the market and help address a shortage.
Liraglutide, sold under the brand name Victoza for diabetes, is an earlier iteration in the same class as semaglutide, the active ingredient in Ozempic. Both are sold by Danish drug giant Novo Nordisk.
The branded drug costs between $500 and $815 per package, depending on the dosage, before discounts or insurance, according to Novo Nordisk, which also sells a version of liraglutide approved for obesity, called Saxenda.
But, Krumholz noted, the newer GLP-1 drugs, which are given as weekly injections instead of daily ones, have shown stronger benefits, especially for patients with obesity – and those aren’t yet available as generics.
Those include Ozempic and its sister drug approved for obesity, Wegovy, which use semaglutide, and Mounjaro and Zepbound, for diabetes and obesity, respectively, which use the active ingredient tirzepatide. They can cost $1,000 a month or more without insurance or discounts.
“This has the possibility of setting up a two-tier system whereby people who can’t afford the more expensive drugs are only able to use drugs with less strong evidence of benefit,” Krumholz said.
Teva Pharmaceuticals, another maker of generic medicines, introduced an authorized generic version of liraglutide in June in the US, under a settlement agreement struck with Novo Nordisk in 2019.
The authorized generic is manufactured by Novo Nordisk, and distributed and sold by Teva. It’s priced at a discount of about 14% to branded Victoza, according to pricing data shared by Erin Fox, a drug shortages and pricing expert at the University of Utah Health.
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Friday from the CNN Health team.
An authorized generic is different from a generic drug, according to the FDA, because it’s the “exact same drug product” as a branded drug, just without the brand name on the label. A generic drug, like the liraglutide generic approved Monday, is a copy of a brand-name drug developed and made by another company.
“Generic drugs provide additional treatment options which are generally more affordable for patients. Today’s approval underscores the FDA’s continued commitment to advancing patient access to safe, effective and high-quality generic drug products,” Dr. Iilun Murphy, the director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research, said in a statement.
It may take a few more manufacturers bringing generics to the market for the price of liraglutide to come down significantly, said Dr. Aaron Kesselheim, a professor of medicine at Harvard Medical School.
Generics are also different from compounded drugs, which are versions made by pharmacies and allowed when medicines are in shortage. Compounded versions, particularly of semaglutide and tirzepatide, flooded the market as those drugs were in shortage over the last few years, although tirzepatide’s shortage was just declared to be resolved. The agency warns that compounded versions are not regulated as closely as generic and branded medicines.
Liraglutide has been in shortage in the US since July 2023, according to an FDA database. The agency, in its Monday announcement, noted it “prioritizes assessment of generic drug applications for drugs in shortage to help improve patient access to these medications.”
Dr. Jody Dushay, an assistant professor of medicine at Harvard Medical School and an attending physician in endocrinology at Beth Israel Deaconess Medical Center, said she hoped generic liraglutide’s price “will be significantly lower than name brand Victoza and Saxenda, but lessons from other drugs that are available as generics teach us that this takes time.”
“I also hope,” Dushay added, “they will get into pharmacies and the hands of patients quickly.”